Medicine and dentistry have long been aware of the connection, association, and direct links between high-risk periodontal pathogens and cardiovascular disease. Knowledge alone has a limited potential to change chronic disease outcomes unless it leads to new clinical practices. Effective and proven new protocols to identify and mitigate virulent periodontal microbes are available today. A small percentage of dentists have implemented these practices and the majority of physicians are unaware of their existence. In the end, one of the significant causes or perpetuators of atherosclerotic vascular disease is left undiagnosed and under treated.
Periodontitis is a polymicrobial, systemic, infectious, and inflammatory disease with genetic expressions. When medical colleagues are faced with an infectious disease, their effective standard of care is to use diagnostic tests to better understand and ultimately treat systemic bacterial diseases. It is based on microbial identification and targeted antimicrobial mitigation of the known pathogens. Periodontitis, perhaps the most prolific human infectious disease, is seemly held to a different standard.
Just over seventy percent of adults over age 65 have symptoms of periodontitis. Over ninety percent of adults with diagnosed heart disease have gums that bleed. The combined prevalence rates of these co-occurring diseases generate large numbers for those keeping statistical databases.
DNA based pathogen testing- a profile of co-occurring microbiomes – oral and arterial
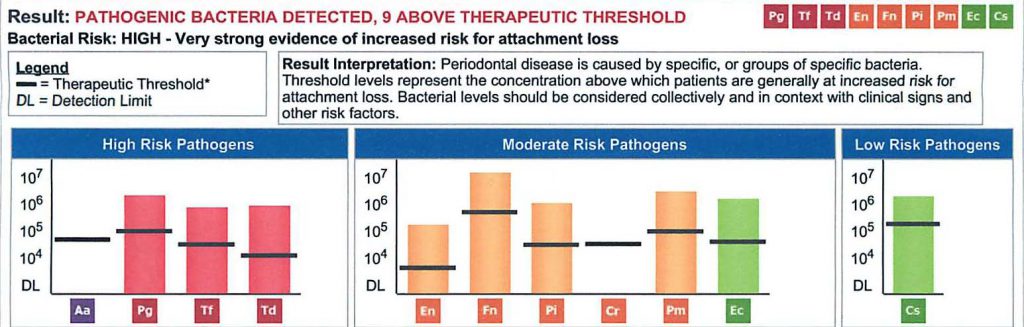
The new standard of care for periodontal diagnosis and treatment planning has been available for about a decade in the United States. DNA based diagnostic salivary testing provides any health care provider specific information regarding the type and concentration of bacteria involved in the disease [fig 1], and supports the achievement of both local and systemic treatment objectives.
The overwhelming majority of published research over the last decade has concluded that high-risk periodontal pathogens are at the epicenter of at least fifty percent or more of cardiovascular events, periodontal disease is an independent risk factor for vascular disease, and all prevention programs should have an oral systemic component.(1,2) In addition, a number of studies have found these microbes to have an increasing or decreasing effect on the intima-media wall thickness of carotid arteries based on their translocated presence or lack thereof.(3)
A landmark study was published in late 2016 showing five periodontal pathogens were causal to atherosclerotic disease and the precise mechanism of causality was described.(4) In early 2017 the Journal of Oral Microbiology reported that the periodontal pathogen Porphyromonas gingivalis was found to be the most abundant single bacterial specie found in coronary and femoral arterial microbiomes.(5)
Finally, the journal Anearobe reported there is a significant correlation between the periodontal pathogen profiles that were found in the same patients when oral biofilms were compared to the arterial biofilms. The study used a group of patients who concurrently expressed periodontitis and vascular disease.(6) The study demonstrated a predictable statistical significance to the co-occurrence of a similar pathogen profile in two different anatomic locations in a given patient.
Below is a salivary pathogen test result called MyPerioPath®. It depicts the presence and level of high-risk pathogens in the mouth as well as a highly predictable picture of pathogens likely present in arterial biofilms.(6) By connecting this specific test result to the ever growing relationship between cardiovascular events and the presence of high-risk periodontal pathogens, it would be reasonable to conclude that if this patient had an cardiac event, there would be a 50% chance it was initiated by one of the specifically identified microbes.(1,2)
Summary
Successful accomplishment of local disease outcomes, as well as now targeting systemic objectives, is now linked to a diagnosis based on the presence of specific microbes. Inadequate therapy based on outdated diagnostic practices has allowed the ongoing translocation of high-risk oral pathogens to become a component of arterial biofilms, micro-biomes, and the progression of arterial disease.
Patients want this standard of care. Physicians will appreciate the level of expertise dentists can add to co-managing chronic disease in their patients when they receive a copy of these reports. Medical doctors must ask dental colleagues to test their high-risk patients. Dentistry must accept the medical model for treating systemic infectious diseases and limit that therapy to high-risk patients.
The traditional standard of diagnostic care embraced by dentistry to date, or lack thereof, has not been adequate. Systemic outcomes have moved to a higher healthcare priority than simply meeting local tissue architecture objectives.
References:
1. Pessi T, Karhunen V, Karjalainen PP, et al. Bacterial signatures in thrombus aspirates of patients with myocardial infarction. Circulation 2013 Mar; 127[11]:1219-1228.
2. Haraszthy VI, Zambon JJ, Trevisan M. Zeid M. Genco RJ. Identification of periodontal pathogens in atheromatous plaque. J Periodontol 2000 Oct; 71[10] 1554-60.
3. Beck JD, Elter JR, Heiss G, Couper D, Mauriello SM, Offenbacher S. Relationship of periodontal disease to carotid artery intima-media wall thickness. Arterioscler Thromb Vasc Biol 2001 Aug;21:1816-22
4. Bale BF, Doneen AL, Vigerust DJ. High-risk periodontal pathogens contribute to the pathogenesis of atherosclerosis. Postgrad Med J 2016 Nov; doi:10.1136/postgradmedj-2016-134279.
5. Mougeot JLC, Stevens CB, Paster BJ, Brennan MT, Lockhart PB, Mougeot FKB. Porphyromonas gingivalis is the most abundant species detected in coronary and femoral arteries. J Oral Microbiol 2017 Feb; 9[1]:1281562.
6. Mahalakshmi K, Krishnan P, Arumugam SB. Association of periodontopathic anaerobic bacterial co-occurrence to atherosclerosis- a cross-sectional study. Anaerobe Feb 2017,44 [suppl C], 66-72.
For more information on how to become an OralDNA Provider – scan HERE: 
- “Words of Inspiration” by Dr. John Kempton - July 7, 2023
- Words of Wisdom from Dr. John Kempton - June 24, 2022
- Establishing New Treatment Objectives for Periodontal Disease - June 22, 2018
